Abstract
Introduction: Transformation of chronic myelomonocytic leukaemia (CMML) to secondary acute myeloid leukaemia (sAML) is frequent and invariably fatal, but poorly characterized. Understanding the transcriptional programs driving progression could reveal therapeutic strategies to prevent/delay transformation. We integrated serial single-cell CITE-seq on CD34+ cells from CMML patients before and after sAML, to evaluate transcriptional and hierarchical dynamics in the hematopoietic stem and progenitor (HSPC) compartment.
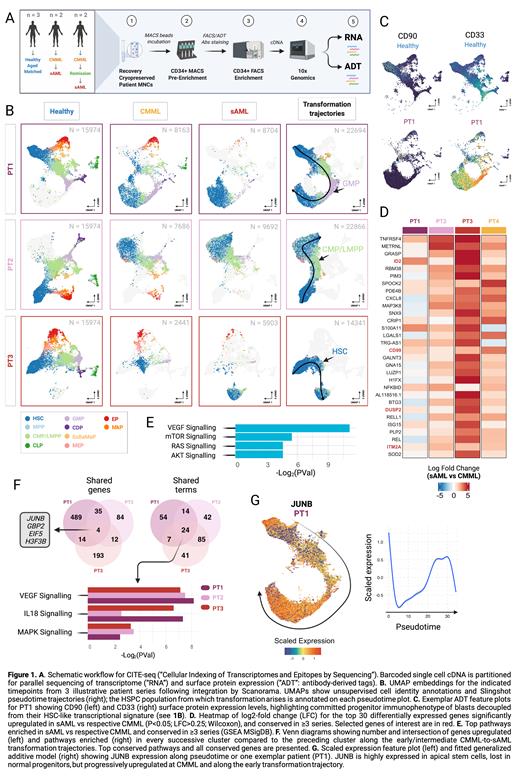
Methods: Four CMML patients with matched bone marrow (BM) from CMML and sAML (± interim remission after azacitidine) were included. Three age-matched healthy controls were sourced from elective hip arthroplasties. Total 13 samples were processed. BM mononuclear cells were thawed and double MACS/FACS-enriched for CD34+ HSPCs. Cells were incubated with 14 HSPC/lineage-defining TotalSeq-A oligonucleotide-conjugated antibodies before parallel analysis of transcriptome and surface protein expression by 10X (Fig 1A).
Results: Total 68,244 HSPCs were analyzed. Controls displayed near-identical UMAP distributions so were pooled for analyses. Optimal UMAP embeddings were generated for each CMML series independently (Fig 1B). UMAP clearly separated populations unique to CMML and sAML samples. Cell identities were inferred by unsupervised annotation from public datasets. CMML samples displayed loss of lymphoid progenitors, but also near-total absence of transcriptionally normal apical HSCs. All patients displayed aberrant expansions of progenitor populations, variously dominated by MPPs, CMPs or GMPs, as validated by antibody-derived tag (ADT) expression patterns.
Pseudotime resolved patient-specific transformation trajectories, with blast expansions emerging from GMP (n=2), CMP/LMPP (n=1) or HSC/MPP (n=1). However, blasts consistently upregulated HSC-like transcriptional programs, in some cases with clear stepwise reversion from committed to stem-like transcriptional states along the computed trajectory (Fig 1B). Accordingly, blasts expressed classical HSC marker genes (ID2, DUSP1, CD52) alongside surface expression of private lineage markers but lacking ADT profiles of apical HSCs (Fig 1C). This decoupling, together with the pseudotime profiles, suggested progressive restoration of an HSC-like signature in downstream progenitors as a consistent feature. Post azacitidine there was marked expansion of megakaryocyte-erythroid progenitors, but despite recovery of normal hematopoiesis no restoration of the depleted apical HSCs. Subsequent sAML represented expansion of pre-existing (treatment-resistant) blasts in 3 case, but emergence of a new, transcriptionally-distinct blast population in the other.
To characterise populations emergent at sAML we evaluated differentially expressed genes (DEG) vs respective CMML counterparts, then intersected gene lists across series. We found 319 genes upregulated in sAML conserved across ≥3 series. Among the most consistently upregulated genes at sAML were DUSP2 and all subunits of ITM2 (notably ITM2A, recently linked to AML). Pathways enriched in sAML included mTOR/PI3K/AKT and VEGF signalling (Fig 1D-E). Also overexpressed were several surface proteins (CD63, CD74, CD82, CD99) also aberrantly upregulated at earlier CMML, suggesting potential as biomarkers to predict/track transformation. Of the sAML signature genes 68 are considered 'druggable', including PIK3R1, NFKBIA and CXCL8. To identify early drivers of transformation we performed unsupervised clustering for each pooled series, comparing DEGs for successive intermediate CMML-to-sAML clusters along the computed trajectories. Common upregulated pathways included VEGF, MAPK and IL18 signaling, with 4 genes, notably the AP1 component JUNB, progressively upregulated during early transformation in all series (Fig 1F-G).
Conclusions: We characterize the repartitioning of HSPC subsets in CMML, including loss of transcriptionally-normal apical HSCs, not restored upon azacitidine response; this may partially explain the transient nature of responses. Cell-of-origin and paths to acute transformation were heterogeneous, but with consistent co-opting of HSC-like programs in lineage-committed blasts. PIK3R1 and JUNB, and the PI3K/AKT/mTOR and VEGF pathways, emerge as candidate mediators of CMML transformation.
Somervaille: Novartis: Consultancy, Honoraria. Wiseman: Novartis: Consultancy; StemLine: Consultancy; Bristol Myers Squibb: Consultancy; Takeda: Consultancy; Astex: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal